Giant Cell Arteritis Increased Risk Of Aortic Dissection?
- Research article
- Open Access
- Published:
Symptomatic aortitis at giant cell arteritis diagnosis: a prognostic factor of aortic result
Arthritis Research & Therapy volume 23, Article number:14 (2021) Cite this commodity
Abstract
Background
Giant cell arteritis (GCA) is frequently associated with aortic involvement that is likely to cause life-threatening structural complications (aneurysm, autopsy). Few studies accept investigated the occurrence of these complications, and no predictive factor has been identified so far. The aim of this report was to investigate factors associated with the risk of aortic complications in a cohort of GCA aortitis.
Methods
Data of all patients managed with aortitis (CT or 18 FDG PET) at the diagnosis of GCA in five hospitals from May 1998 and April 2022 were retrospectively nerveless. Clinical features were compared according to the presence of aortitis symptoms. The predictive factors of occurrence or aggravation of aortic structural abnormalities were investigated.
Results
One hundred and seventy-one patients with GCA aortitis were included; 55 patients (32%) had symptoms of aortitis (dorsal/lumbar/abdominal pain, aortic insufficiency) at diagnosis. The median follow-upwards was 38 months. Aortic complications occurred later on a median fourth dimension of 32 months. There were 19 new aortic aneurysms or complications of aneurysm and 5 dissections. Survival without aortic complexity was significantly unlike between the symptomatic and non-symptomatic groups (Log rank, p = 0.0003). In multivariate analysis the presence of aortitis symptoms at diagnosis (Hour 6.64 [one.95, 22.6] p = 0.002) and GCA relapse (Hr iii.62 [1.2, ten.9] p = 0.02) were factors associated with the occurrence of aortic complications.
Conclusion
In this study, the presence of aortitis symptoms at the diagnosis of GCA aortitis and GCA relapse were contained predictive factors of occurrence of aortic complications during follow-up.
Introduction
Giant jail cell arteritis (GCA) is the most frequent systemic vasculitis, in which aortitis is present at diagnosis in xl to 65% of cases [1, two]. The screening for aortitis is now consensually included in international guidelines for all patients diagnosed with GCA [iii, 4]. Aortic interest is not systematically evaluated, although it is a potentially life threatening condition due to aortic complications such as aortic aneurysms and dissection [five,vi,7,8,9]. Aortic complications may be nowadays at the time of diagnosis but may likewise occur many years after the diagnosis of GCA aortitis [9].
There are few data on the prognostic value of aortic involvement in GCA. Overall mortality does not announced to be increased [x, 11]. In contrast, in a cohort study of 204 GCA patients with large vessel interest, survival was impaired in patients with aortic aneurysm or dissection [12]. Moreover, large vessel impairment was reported to be associated to higher GCA relapse rates [10, 11, 13, fourteen].
To date, there are no articulate information on the link between clinical presentation of aortitis, at the fourth dimension of diagnosis of GCA, and the prognosis of the disease. The aim of this study was to evaluate clinical presentation and outcome of GCA-related aortitis.
Methods
Patients
This study included patients diagnosed with GCA between May 1998 and Apr 2022 from v hospitals in western French republic. Data were collected from medical records. Each patient had to meet at least three American Higher of Rheumatology criteria for the diagnosis of GCA [15]; or be over 50-year-old age with a biological inflammatory syndrome and with the presence of an aortic inflammatory illness on imaging exam (CT, MRI, PET) [3]; and have an aortic interest at diagnosis defined by the following: a circumferential aortic parietal thickening > 2.2 mm on CT/MRI, and/or a grade 2 or iii parietal aortic hypermetabolism on PET [3, 16].
Symptomatic aortitis was defined by the presence 1 month before or at GCA diagnosis of ane or more than of the following signs: recent, less 2 months, occurrence of chest, dorsal, lumbar, or abdominal pain; or unknown aortic insufficiency with recent dyspnea highlighted at aortitis diagnosis. This signs were unexplained by any other crusade than aortitis (musculoskeletal degenerative disease, atherosclerotic or other aortic disease).
Ethics
This written report accept received ethics board approving by GNEDS (Groupe Nantais d'Ethique et de Soins) the local ideals committee of the Academy Hospital of Nantes (20200219). Each patient included in this written report received written data, and no patient objected to this written report.
Definition of study end-points
Aortic complexity was defined by the occurrence, at least 1 month after initial imaging of the aorta, of a new aortic structural aberration (aneurysm: thoracic aortic diameter > 4 cm or abdominal aorta diameter > three cm; or aortic autopsy), or the demand for aortic surgery in response to a threatening structural abnormality (aortic dissection, progression of the aneurysm reaching a critical size, or aortic insufficiency).
Peripheral vascular consequence was defined by the occurrence, at least one calendar month subsequently initial imaging, of one of the post-obit complication: limb ischemia, mesenteric or renal ischemia, myocardial ischemia, or ischemic stroke.
GCA relapse was defined past the concomitant reappearance of GCA-related clinical manifestations and a biological inflammatory syndrome (CRP ≥ 15 mg/l), or the reappearance of a biological inflammatory syndrome with inflammatory arterial parietal changes on CT, MRI or PET [17,xviii,xix,20].
Patients with not-symptomatic aortitis (noS-Ao) at diagnosis were compared to patients with symptomatic aortitis at diagnosis (South-Ao).
Only radiographically monitored patients were included in the multivariate study and survival assay.
Statistical analysis
Qualitative values were expressed in terms of numbers and percentages. The mean comparisons were made using t test. Frequency comparisons were made by a chi-squared exam or the Fisher examination co-ordinate to the statistical headcount. Prognostic factors associated with aortic complication were evaluated with Cox models. Hazard ratios (HR) with their 95%CI has been estimated as clan measures. Variables with p < 0.05 in univariate model and all the variables already known to be misreckoning factors were candidate variables for multivariate model. Survival curves were estimated with their 95% confidence interval (95%CI) using Kaplan-Maier estimators, and Log rank tests were performed to compare aortic complication gratuitous survival between groups.
Results
Characteristics of the 171 patients with GCA aortitis
This study included 171 cases of GCA with aortitis at diagnosis, whose characteristics are described in Table i. Aortic CT was performed at baseline in 142 patients (83%), both CT and PET in 67 (39%), PET in 92 (54%), and aortic MRI in 12 (7%). Ascending thoracic aorta was involved in 123 cases (72%), aortic arch in 107 (63%), descending thoracic aorta in 110 (64%), and abdominal aorta in 95 (56%). Thoracic aortic aneurysm at GCA diagnosis and intestinal aortic aneurysm was present respectively in 13 patients (23.6%) in S-Ao, ix (seven.viii%) in noS-Ao (p = 0.009), 6 (10.9%) in S-Ao, and iv (3.iv%) in noS-Ao (p = 0.08).
Aortitis was symptomatic in 55 cases (32%), including 51% chest pain, 31% abdominal pain, 16% back hurting, and 35% had previously unknown aortic insufficiency with recent dyspnea.
Ten patients (five.viii%) had undergone aortic surgery for an inaugural complication: 5 Stanford-A aortic dissections (1 patient died), and five ascending thoracic aorta aneurysms (all underwent surgery because of the size of the aneurysms, of which three were also responsible for severe aortic insufficiency).
Immunosuppressive handling was introduced in eighteen.1% (n = 31) of cases (12.vii% in S-Ao and xx.vii% in noS-Ao (p = 0.20)): methotrexate in 23 cases, tocilizumab in three cases, azathioprine in 3 cases, and cyclophosphamide in 2 cases.
At least one control imaging (CT angiography, PET, or MR angiography) was performed for 98 patients: 44 (80%) of S-Ao patients and 54 (52%) of noS-Ao patients. For patients who had a control imaging during follow-up, mean duration betwixt initial and last imaging was 35.6 months (± 34.7), mean follow-up time was 34.4 months (± 30.8) for S-Ao patients and 36.five months (± 37.ix) for noS-Ao patients (p = 0.77).
Twenty-three aortic complications occurred during follow-upward (23.5% of patients with command imaging): 15 in S-Ao patients (34.1% with command imaging) and 8 in noS-Ao patients (14.eight% with command imaging) (p < 0.01); these complications do non include aortic aneurysms, dissections or surgeries that occurred at diagnosis or in the month following the diagnosis of GCA. There were 15 new aortic aneurysms, 4 disquisitional increase size of a pre-existing aneurysm requiring surgery, and iv aortic dissections: 3 (v.5%) in S-Ao and 1 (0.9%) in noS-Ao (p = 0.06).
Aortic complications occurred afterward a median delay of 27 months [2 to 101 months]. I patient died following the rupture of an abdominal aortic aneurysm that was not present at diagnosis. These aortic complications required surgery for 9 patients, while iii other had contraindications for surgery because of their health status, and 1 declined surgery. Of the 15 aortic aneurysms that occurred during the follow-up, 11 were located on the ascending thoracic aorta, and 4 on the abdominal aorta.
Vascular evolution and relapse in GCA aortitis patients
Multivariate cox model (Table 2) showed that the presence of aortitis symptoms at diagnosis (HR 6.64 [1.95, 22.6] p = 0.002) and GCA relapse (HR 3.62 [1.2, 10.9] p = 0.02) were contained factors of aortic complication.
Survival without aortic complexity was significantly different between the symptomatic and not-symptomatic groups (Log rank, p = 0.0003) (Fig. 1).
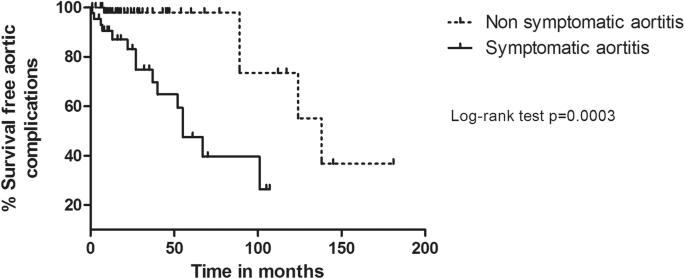
Survival without aortic complication: comparison according to the presence of aortitis symptoms at GCA diagnosis on radiographically monitored patients
Nineteen (35%) Due south-Ao and 53 (46%) noS-Ao patients had a GCA relapse (p = 0.18); 8 (xv%) S-Ao and 21 (18%) noS-Ao patients developed subsequent peripheral vascular event (p = 0.72). Eighteen patients died during follow-upwards (2 S-Ao and 16 noS-Ao, p = 0.07). Causes of death recorded were 1 dissection of the thoracic aorta, 1 rupture of an abdominal aortic aneurysm, 5 cardiovascular causes, 3 infections, 1 cancer, and 1 severe hemorrhage.
Discussion
This nowadays report is the kickoff aimed to appraise the prognostic bear upon of symptomatic aortitis in a large cohort of GCA patients with well-documented aortitis at diagnosis.
Patients with symptomatic aortitis had significantly more than cardiovascular risk factors (smoking, hypertension) than asymptomatic patients, suggesting a potential boosted consequence leading to astringent aortic involvement. In accordance to the literature, the aorta was mostly affected in its thoracic segment, especially the ascending thoracic aorta [1, 2]. Aortic aneurysms may be nowadays at the time of diagnosis of GCA, concerning between 4 and 23% of patients [1, 2]. In our nowadays written report, exclusively including patients with well-documented aortitis, this frequency was quite high (19%). At diagnosis or during follow-up, de Boysson et al. reported the discovery of aortic aneurysms in 11% of patients with aortitis, only also in 7% of GCA patients without aortitis [11]. In this study, an aortic aneurysm or dissection was inaugural in 23.4% of patients, with a need for surgery at diagnosis in v.viii% of all patients. These data support the involvement of performing aorta imaging at diagnosis of GCA [4].
In our experience, patients with non-symptomatic aortitis received more frequently Methylprednisolone pulses. This is in function due to a higher frequency of ocular involvement in these patients. The utilise of immunosuppressive treatment during follow-up remained infrequent (18.1% of all cases) and mainly used for severe or resistant forms.
In case of aortitis, control imaging should be used to assess evolution and discover structural complications. The lack of systematic monitoring probably leads to an underestimation of the actual number of aortic complications. In the presence of aortitis, imaging at 1 and ii years from the diagnosis may be considered to assess wall inflammation and screen for aortic aneurysms; afterwards imaging can be discussed in instance of multiple relapses or corticosteroid dependence.
During GCA, possible predictive factors of aortic complication (aneurysm, autopsy) accept already been suggested: coronary artery disease and hypercholesterolemia [7, 12], arterial hypertension and PMR at diagnosis [21], and presence of aneurysms of the subclavian arteries [22], but prospective studies are lacking to assess this of import effect. In a study including 549 patients, aortic inflammation has been described equally the best predictor of aortic dilation [11]. In our own previous study, aortitis at the diagnosis of GCA was associated to higher corticosteroid dependence and college cardiovascular mortality [8].
In this study, 23.v% of patients (14.8% of noS-Ao patients and 34.ane% of S-Ao patients) adult an aortic complication after a median filibuster of 27 months, which is quite similar to the 2.5 years constitute in the historical study past Evans et al. [5]. Moreover, we plant that symptomatic aortitis at diagnosis was associated to a significantly higher adventure of developing aortic complications, with hazard ratio at half-dozen.64. The presence of aortitis symptoms could therefore reflect a disease with locally more severe inflammation that is the basis for structural damage. The interpretation of the prognosis of symptomatic aortitis must exist careful considering it also tin can be argued that advanced aortic affliction at GCA diagnosis has unfavorable prognosis due to a significant diagnostic delay or a prior aortic disease.
This study presents an original concept with the search for clinical signs of aortitis at GCA diagnosis. If these results are confirmed, imaging could aid identify patients with more than aggressive aortitis.
Our study has several limitations: with the potential data capture errors and missing data that are inherently an issue with all retrospective data collection and brusk median follow-upwardly. Although the initial dose of corticosteroid therapy was the aforementioned in both groups, tapering schedules were not standardized, and the apply of immunosuppressive therapy was not standardized. Moreover, for nearly patients, control modalities and blood pressure objectives were not clearly indicated. Only 18.1% of the accomplice were treated with additional immunosuppression despite recent EULAR recommendations [23] since this is a cohort with patients included over a long period with some patients exclusively managed with steroids. The follow-up time limited the ability to capture aortic complexity that tin can occur 5–10 years after diagnosis.
In improver, clinical signs associated with symptomatic aortitis are non-specific: hurting or dyspnea is very frequent in elderly population; however in this study, symptomatic aortitis was retained after exclusion of musculoskeletal degenerative affliction and atherosclerotic or other aortic disease that could explicate these symptoms.
Several aortic CT scans performed were non synchronized to the heart rate (limiting the assay of the ascending thoracic aorta) and some had no late arterial phase to evaluate aortic parietal contrast; follow-upwards imaging was not systematic and was performed at widely varying times.
If these information are confirmed in prospective studies, a more intensive initial treatment for symptomatic aortitis at diagnosis—with initial corticosteroid therapy combined with immunosuppressive therapy—could be evaluated.
The management of cardiovascular take a chance factors is too fundamental, including blood pressure monitoring with self-measures, a strict goal for LDL cholesterol level, smoking cessation, and regular concrete activeness.
Conclusion
Aortitis in GCA may lead to severe complications like aneurysm or dissection. Patients who have symptomatic aortitis, mainly chest and intestinal pain, at the time of diagnosis of GCA, could represent a singled-out sub-group of aortitis with more aortic aneurysm or autopsy during follow-up than patients with non-symptomatic aortitis. Prospective studies are needed to ostend our results. In society to take better care of these patients, recommendations on the frequency and modalities of aortic imaging remain to be defined.
Availability of data and materials
Data are available to request. No expiration date of data requests is currently set in one case they are made available. Access is provided after a proposal has been canonical by an independent review committee identified for this purpose and after receipt of a signed information sharing understanding. Data and documents, including the study protocol, statistical analysis programme, and clinical study report, will be provided in a secure data sharing surroundings for upwards to 2 years per proposal. For details on submitting a request, see the instructions provided at www.clinicalstudydatarequest.com.
Abbreviations
- CT:
-
Computed tomography
- GCA:
-
Behemothic jail cell arteritis
- noS-Ao:
-
Non-symptomatic aortitis
- PET:
-
Positron emission tomography
- Southward-Ao:
-
Symptomatic aortitis
References
-
Agard C, Barrier J-H, Dupas B, Ponge T, Mahr A, Fradet G, et al. Aortic interest in recent-onset giant cell (temporal) arteritis: a example-control prospective written report using helical aortic computed tomodensitometric scan. Arthritis Rheum. 2008;59:670–vi.
-
Prieto-González S, Arguis P, García-Martínez A, Espígol-Frigolé G, Tavera-Bahillo I, Butjosa Chiliad, et al. Large vessel interest in biopsy-proven giant jail cell arteritis: prospective study in 40 newly diagnosed patients using CT angiography. Ann Rheum Dis. 2022; [cited 2022 Jan 21]; Bachelor from: http://ard.bmj.com/content/early/2012/01/20/annrheumdis-2011-200865.abstract.
-
Dejaco C, Ramiro S, Duftner C, Besson FL, Bley TA, Blockmans D, et al. EULAR recommendations for the utilise of imaging in large vessel vasculitis in clinical do. Ann Rheum Dis. 2022;77:636–43 BMJ Publishing Group Ltd.
-
Bienvenu B, Ly KH, Lambert Yard, Agard C, André M, Benhamou Y, et al. Direction of behemothic cell arteritis: recommendations of the French Report Group for Large Vessel Vasculitis (GEFA). Rev Méd Interne. 2022;37:154–65.
-
Evans JM, Bowles CA, Bjornsson J, Mullany CJ, Hunder GG. Thoracic aortic aneurysm and rupture in giant cell arteritis. A descriptive study of 41 cases. Arthritis Rheum. 1994;37:1539–47.
-
Agard C, Ponge T, Fradet Thou, Baron O, Sagan C, Masseau A, et al. Behemothic cell arteritis presenting with aortic dissection: two cases and review of the literature. Scand J Rheumatol. 2006;35:233–6.
-
Nuenninghoff DM, Hunder GG, Christianson TJH, McClelland RL, Matteson EL. Incidence and predictors of large-avenue complication (aortic aneurysm, aortic dissection, and/or large-avenue stenosis) in patients with giant jail cell arteritis: a population-based report over 50 years. Arthritis Rheum. 2003;48:3522–31.
-
de Boysson H, Liozon E, Espitia O, Daumas A, Vautier M, Lambert Thousand, et al. Different patterns and specific outcomes of large-vessel involvements in giant cell arteritis. J Autoimmun. 2022;103:102283.
-
Espitia O, Samson G, Le Gallou T, Connault J, Landron C, Lavigne C, et al. Comparison of idiopathic (isolated) aortitis and behemothic cell arteritis-related aortitis. A French retrospective multicenter study of 117 patients. Autoimmun Rev. 2022;xv:571–6.
-
Espitia O, Néel A, Leux C, Connault J, Espitia-Thibault A, Ponge T, et al. Behemothic cell arteritis with or without aortitis at diagnosis. A retrospective study of 22 patients with longterm followup. J Rheumatol. 2022;39:2157–62.
-
de Boysson H, Daumas A, Vautier Yard, Parienti J-J, Liozon E, Lambert Yard, et al. Large-vessel involvement and aortic dilation in giant-cell arteritis. A multicenter study of 549 patients. Autoimmun Rev. 2022;17:391–8.
-
Kermani TA, Warrington KJ, Crowson CS, Ytterberg SR, Hunder GG, Gabriel SE, et al. Large-vessel involvement in giant prison cell arteritis: a population-based cohort study of the incidence-trends and prognosis. Ann Rheum Dis. 2022;72:1989–94.
-
Muratore F, Kermani TA, Crowson CS, Green AB, Salvarani C, Matteson EL, et al. Big-vessel behemothic cell arteritis: a cohort written report. Rheumatol Oxf Engl. 2022;54:463–70.
-
Dumont A, Parienti J-J, Delmas C, Boutemy J, Maigné G, Martin Silva Due north, et al. Factors associated with relapse and dependence on glucocorticoids in giant cell arteritis. J Rheumatol. 2022;47:108–16.
-
Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The American College of Rheumatology 1990 criteria for the nomenclature of Takayasu arteritis. Arthritis Rheum. 1990;33:1129–34.
-
Enfrein A, Espitia O, Bonnard G, Agard C. [Aortitis in giant cell arteritis: diagnosis, prognosis and treatment]. Presse Med Paris Fr 1983. 2022;48:956–67.
-
Kermani TA, Warrington KJ, Cuthbertson D, Carette S, Hoffman GS, Khalidi NA, et al. Disease relapses among patients with giant cell arteritis: a prospective, longitudinal cohort study. J Rheumatol. 2022;42:1213–7.
-
Martinez-Lado L, Calviño-Díaz C, Piñeiro A, Dierssen T, Vazquez-Rodriguez TR, Miranda-Filloy JA, et al. Relapses and recurrences in giant cell arteritis: a population-based study of patients with biopsy-proven disease from northwestern Spain. Medicine (Baltimore). 2022;90:186–93.
-
Restuccia Thou, Boiardi L, Cavazza A, Catanoso M, Macchioni P, Muratore F, et al. Flares in biopsy-proven giant cell arteritis in Northern Italy: characteristics and predictors in a long-term follow-up study. Medicine (Baltimore). 2022;95:e3524.
-
Alba MA, García-Martínez A, Prieto-González South, Tavera-Bahillo I, Corbera-Bellalta M, Planas-Rigol E, et al. Relapses in patients with giant cell arteritis: prevalence, characteristics, and associated clinical findings in a longitudinally followed accomplice of 106 patients. Medicine (Baltimore). 2022;93:194–201.
-
Gonzalez-Gay MA, Garcia-Porrua C, Piñeiro A, Pego-Reigosa R, Llorca J, Hunder GG. Aortic aneurysm and dissection in patients with biopsy-proven behemothic jail cell arteritis from northwestern Kingdom of spain: a population-based study. Medicine (Baltimore). 2004;83:335–41.
-
Muratore F, Kermani TA, Crowson CS, Koster MJ, Matteson EL, Salvarani C, et al. Large-vessel dilatation in behemothic cell arteritis: a different subset of disease? Arthritis Care Res. 2022;70:1406–eleven.
-
Hellmich B, Agueda A, Monti S, Buttgereit F, de Boysson H, Brouwer Eastward, et al. 2022 update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2022;79:19–30.
Funding
No specific funding was received from any funding bodies in the public, commercial, or not-for-turn a profit sectors to carry out the work described in this manuscript.
Author information
Affiliations
Consortia
French Written report Group for Big Vessel Vasculitis (GEFA)
Contributions
GB: acquisition of information; analysis and interpretation of data; drafting of the manuscript. GU, CL, JC, CL, PR, FM, AA, MA, CD, MH, JM: acquisition of data and critical review; BG: methodology; CA analysis and interpretation of information OE: written report concept and blueprint; conquering of data; analysis and interpretation of information; drafting of the manuscript; supervision. All authors read and approved the final manuscript.
Respective writer
Ethics declarations
Ethics approval and consent to participate
This report accept received ethics board approval past GNEDS (Groupe Nantais d'Ethique et de Soin) the local ethics committee of the University Infirmary of Nantes (20200219).
Consent for publication
Each patient included in this study received written information and no patient objected to this study.
Competing interests
The authors accept declared no conflicts of interest.
Boosted information
Publisher's Notation
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in whatsoever medium or format, every bit long as you give appropriate credit to the original writer(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party fabric in this article are included in the article's Artistic Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will demand to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/naught/1.0/) applies to the data fabricated available in this article, unless otherwise stated in a credit line to the data.
Reprints and Permissions
About this article
Cite this article
Espitia, O., Blonz, Grand., Urbanski, G. et al. Symptomatic aortitis at giant cell arteritis diagnosis: a prognostic factor of aortic result. Arthritis Res Ther 23, 14 (2021). https://doi.org/10.1186/s13075-020-02396-5
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/s13075-020-02396-five
Keywords
- Behemothic cell arteritis
- Aortitis
- Aneurysm
- Aortic dissection
- Prognosis
Giant Cell Arteritis Increased Risk Of Aortic Dissection?,
Source: https://arthritis-research.biomedcentral.com/articles/10.1186/s13075-020-02396-5
Posted by: davismoomple.blogspot.com


0 Response to "Giant Cell Arteritis Increased Risk Of Aortic Dissection?"
Post a Comment